Welcome to the Williams Lab

Our lab is focused on the study of protein macromolecular complexes involved in epigenetic regulation of gene expression. Currently we study the methylcytosine binding domain 2 (MBD2) protein which recruits the Nucleosome Remodeling and Deacetylase (NuRD) complex to methylated DNA and silences expression of the associated gene.
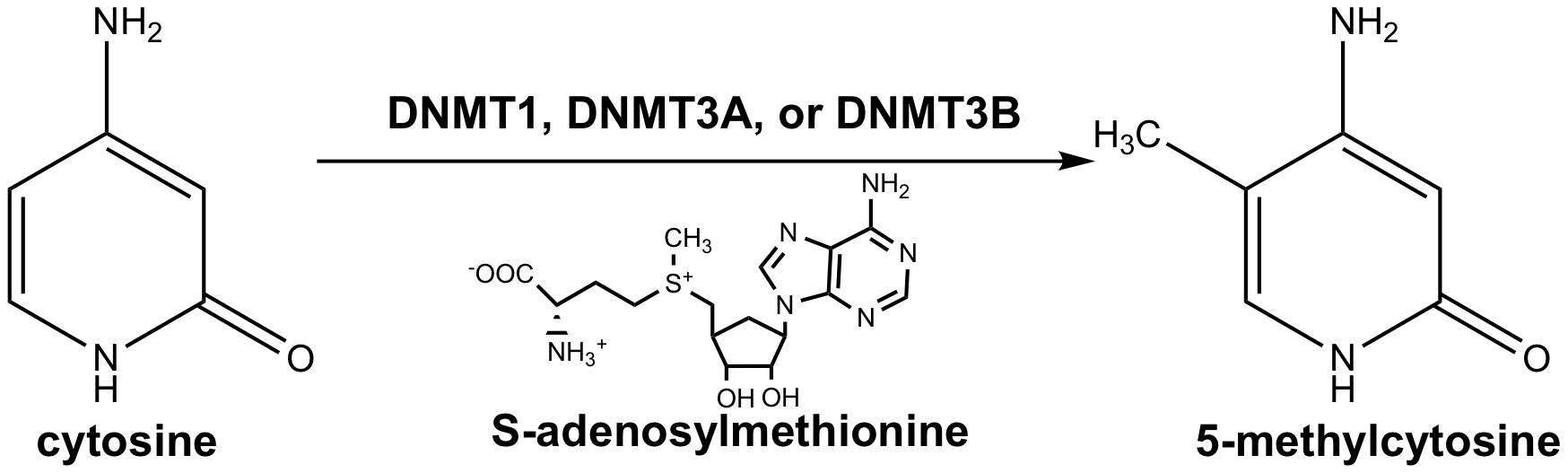
DNA methylation involves the addition of a methyl group to the C5 position of a cytosine base. This
modification occurs in the context of CpG dinucleotides in double-stranded DNA in which the
symmetrically related cytosines are methylated. This modification can be maintained across cell division
since each daughter cell inherits one of the methylated strands.
The NuRD complex
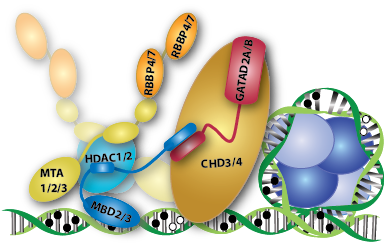
The NuRD complex comprises MBD2 or MBD3 and at least five additional proteins, each of which have multiple
isoforms: HDAC1/2, RBBP4/7, MTA1/2/3, GATAD2A/B, and CHD3/4. We have been focusing our efforts on
understanding i) how the MBD2/3 proteins bind and distribute on DNA and ii) how they interact with other
members of the complex.
isoforms: HDAC1/2, RBBP4/7, MTA1/2/3, GATAD2A/B, and CHD3/4. We have been focusing our efforts on
understanding i) how the MBD2/3 proteins bind and distribute on DNA and ii) how they interact with other
members of the complex.
MBD2:DNA complex
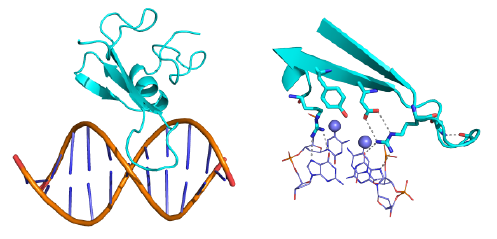
Methylcytosine binding domains (MBD) recognize symmetrically related methylcytosines through a small (~60 amino acid) domain. At least five members of the MBD family are present in mammals (MeCP1, MBD1-4). The most ancient members of the MBD family, MBD2 and MBD3, recruit the NuRD complex in a mutually exclusive manner.
GATAD2A-MBD2 complex
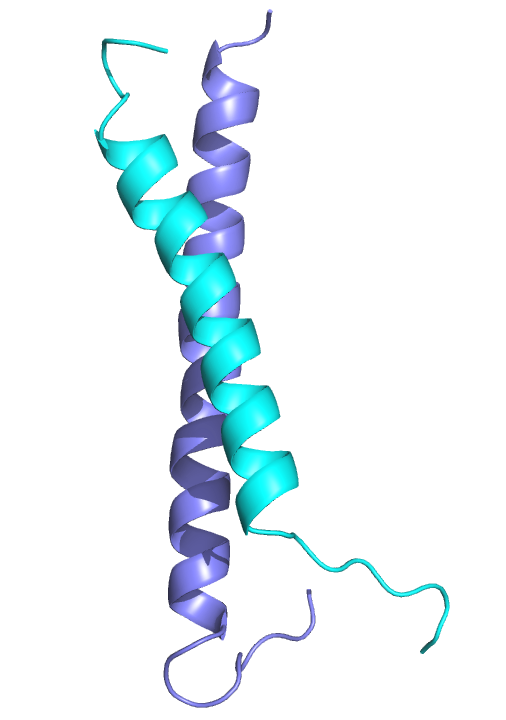
A coiled-coil interaction between the GATAD2A and MBD2 proteins recruits the CHD3/4 nucleosome remodeler to the NuRD complex. We solved the structure of this interaction and have shown that a small peptide inhibitor blocks methylation dependent gene silencing. Based on this work, we are currently developing an inhibitor of complex formation to restore expression of methylated and silenced genes.
NMR – 2D 15N-HSQC
We use nuclear magnetic resonance (NMR) spectroscopy and other biophysical techniques including isothermal titration calorimetry, circular dichroism and surface plasmon resonance to investigate structure and dynamics of protein-protein and protein-DNA complexes.